About Gene Therapy & Viral Vectors In NC
NC-VVIRAL is coalescing a broad multidisciplinary expertise and background IP across the Universities in the Research Triangle Park, and is assembling an industrial advisory board of global biopharmaceutical companies and technology vendors
Our Mission
Viral vectors are a new and exciting class of products that are transforming medicine and biotechnology, from gene therapies targeting rare diseases, oncolytic viruses for cancer therapy, and viral vaccines – to CRISPR-Cas carriers for plant and animal engineering. In recent years, the demand for viral vectors with high potency, purity, and safety has grown exponentially, putting significant pressure on the industrial pharmaceutical and biomanufacturing communities. Furthermore, biomanufacturing aims to be sustainable and affordable, and adapt flexibly across a wide range of production scales – from small doses for clinical and research applications to the massive roll-out of viral vaccines.
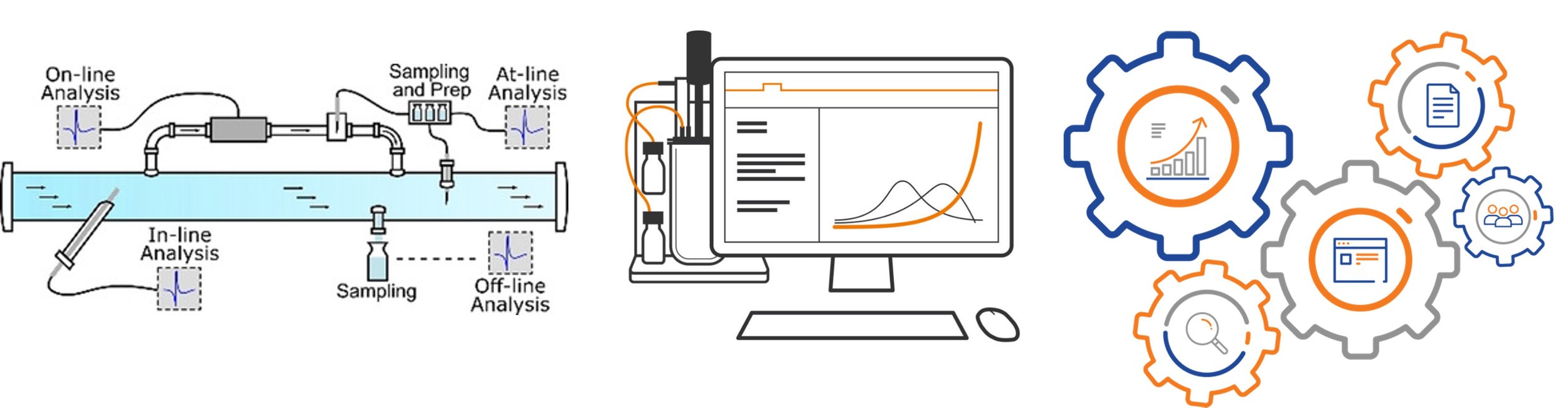
There is consensus in the engineering community that achieving these goals will rely on introducing (i) novel upstream and downstream unit operations as well as on/in-line sensors that are tailored to viral vectors; (ii) transformative process designs that enable rapid, continuous, reconfigurable, and automated production; and (iii) a massive influx of skilled workers with fundamental and hands-on training focused on the production and analytical characterization of viral vectors.
Responding to these challenges, the mission of NC-VVIRAL is to establish a Foundry of Viral Vector Manufacturing Technologies including:
-
Current IP portfolio licensable to partners: a suite of novel biomanufacturing and biosensing tools, data science resources, and bioprocess layouts has been established in NC-VVIRAL and is available to industrial partners in the form of licensable IP, data repositories, blueprints, and prototypes for beta testing.
-
Bespoke solutions for industries and researchers: the NC-VVIRAL scientific and engineering team works closely with industrial and academic partners to develop customized solutions serving viral vector expression or purification needs, analytical characterization, process development and optimization.
-
Training modules and an workforce-ready cohort: in partnership with the Biomanufacturing Training and Education Center (BTEC), NC-VVIRAL provides demonstrations on bioprocess and analytical equipment and training modules on viral vector manufacturing; a cohort of postdoc, PhD-, MS-, and BS-level graduates with strong fundamental and hands-on experience is also being trained to enter the regional workforce.

Innovations in Viral Vector Manufacturing
Bioprocess Tools
We aim to boost product efficacy and safety, reducing process footprint, time, and cost by developing single-use adsorbents that capture process- and product-related impurities and fractionate the product stream in straight-through mode to isolate the active vectors loaded with the therapeutic transgene.

Sensors & Automation
To realize real-time process control for the improvement of manufacturing efficiency and product quality, we are developing sensors and microsystems that process and analyze samples from bioreactors or the purification pipeline to track viral vector titer, full:empty ratios, transduction and other product and process Critical Quality Attributes.